Communities and conservation
When I was younger, I used to love visiting our local creek: it was a beautiful spot of nature a short walk from home. On a couple occasions, my Dad took me to the creek to catch yabbies – for a suburban kid, it was one of the few times I actually held and interacted with wild biodiversity, and helped foster my love for conservation and inquiry into biology. In the late 2000s to early 2010s, a likely combination of local pollution and extensive drought extirpated the yabbies from the creek – I would never see one in that creek again. I was devastated for the local loss of a fascinating creature, and the connection to nature it represented, but felt powerless to remedy the situation. To my knowledge, there are still no yabbies in that creek.

I suspect this experience is not unique – species impacts across the globe have resulted in countless local extirpations, and many communities feel saddened by their absence. But as ICTC2023 highlighted late last year, conservation translocations (and reintroductions) have come a long way in recent years – restoring lost local populations is not an unfeasible proposition. But despite their importance for the conservation of threatened species – and the direct link to the communities which will inevitably support, foster, and connect to these restored populations – relatively few reintroduction programs involve local communities. Instead, these “citizen science” or “community science” programs are often relegated to aspects of monitoring or data collection for research institutions, playing a somewhat passive role in meaningful conservation action.
Why aren’t there more community-led reintroductions?
Reintroductions, as you might expect, are a complex affair: they require extensive resources, expertise, and ongoing support to be most effective. Particularly, designing and implementing a reintroduction program requires some critical evidence for decision-making. For example, we often need to consider:
- Where are we collecting individuals from to source our reintroduced populations?
- How many are we taking? Should we be taking older or younger animals? Even males and females, or a skewed ratio? How many can be reliably taken from our chosen source without impacting it?
- What threats need to be managed at the reintroduction site, to make sure it will persist in the long-term?
- How do we best maintain genetic diversity in the reintroduced population? Do we need to consider the impact of population structure if using multiple sources?
This last topic is particularly challenging for non-geneticists to answer. Studies have shown that maintaining genetic diversity in a reintroduced population is a key component of its long-term persistence, and we can miss critical information by omitting genetic assessments. But collaborations between local communities, conservation geneticists (such as from an academic institution) and on-the-ground environmental managers are exceedingly rare. Together, these observations paint a daunting picture for the challenges of a community-led reintroduction program…
The Magnificent Six
…but our recently published study shows it’s not impossible! Our paper – published in Conservation Science and Practice – demonstrates how a collaboration between local councils, environmental groups, natural resource agencies, fish hobbyist groups and conservation geneticists (that’s me!) helped to reintroduce the southern pygmy perch to the Bendigo region in Victoria, Australia.

This project was proposed by the Tri-State Murray Natural Resource Management (NRM) Alliance – as the name suggests, a collaboration of six regional bodies across three states. The Alliance aims to “grow local economy, secure the environment, and motivate the community” along the Murray River corridor, including revitalising the local environment. A core component of this aim is the “Magnificent Six” project, which aims to recover or locally restore populations of six, small native fish species (including southern pygmy perch) through habitat recovery and translocation efforts. Our specific program focused on the reintroduction of southern pygmy perch into the Bendigo region, through a collaborative working group of local councils (City of Greater Bendigo; CoGB), environmental groups (Friends of Crusoe and No. 7 Reservoir), natural resource agencies (North Central Catchment Management Authority; NCCMA), fish hobbyist groups (Native Fish Australia; NFA, and the Australian and New Guinea Fishes Association) and conservation geneticists (Molecular Ecology Lab at Flinders University; MELFU). Ultimately, the goal was to secure wild populations of southern pygmy perch from catchments within the Bendigo region, captively breed broodstock, and translocate offspring into nearby restored habitats: at each stage, we also collected and analysed genetic data to guide some management decisions and assess the outcome of the reintroduced populations.
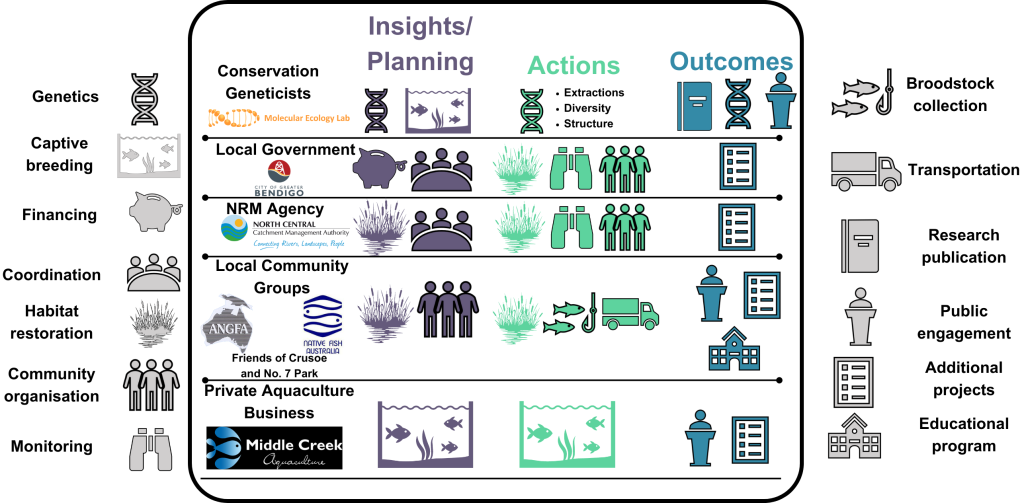
Bringing back the southern pygmy perch
Where did they come from?
In 2018, after consultation with local communities, a partnership between the NCCMA, CoGB Council, NFA and ANGFA was established with an action plan for the southern pygmy perch. Potential source sites were selected based on the prior knowledge of population genetic structure from MELFU research and pre-translocation surveys by locals. Three nearby locations were chosen – two adjacent to the Campaspe River (McIvor Creek and Jew Harp Creek) and one in the Avoca River – as suitable sources for the breeding program. In September 2018, volunteer community members – recruited through NFA and ANFGA, and under the guidance of natural resource managers – travelled to each of these sites to collect the wild fish. These champion volunteers then helped transport them to the nearby aquaculture facility at Middle Creek Farm – a private aquarium business which facilitated the breeding program for these endangered fish. As only males were caught from McIvor Creek, these were pooled with the Jew Harp Creek individuals into the same tank (you’ll see why that’s important to note later). Thus, two “lineages” (referred to as the “Campaspe” and “Avoca” lineages) were bred separately at Middle Creek Farm. Based on our (MELFU) previous experience with captive-breeding and reintroduction of southern pygmy perch, we provided some advice for how best to set up the breeding arrangements to maximise the retention of genetic diversity and numbers in the captive-breeding population. Throughout 2019, a single new generation (“F1”) was produced by the program, with hundreds of new fish raised and grown ready to be released into their new home. Local volunteers also contributed an immense effort to looking after the broodstock and fry and were involved in their subsequent releases.

Where did they go?
While the breeding program was happening, the CoGB Council and the Friends of Crusoe and No. 7 Reservoir group prepared three proposed release sites (two within Cadella Way wetlands and one in No. 7 Frog Ponds) through large-scale aquatic vegetation planting and woody habitat installation to generate suitable micro-habitat for the incipient fish. They also ensured that none of the main threats to pygmy perch – particularly invasive redfin perch and European carp – were present in the target sites. In January 2020, a new generation of captively-bred pygmy perch were ready to be released: at each site, 200 fish (mostly originating from the Campaspe lineage due to numbers) were released. But before we let them go, we made sure to collect DNA samples from a subset of these individuals to assess how the captive-breeding program had affected genetic diversity and inbreeding. Throughout the year, the fish were allowed to adjust and thrive in their new wild homes: in September 2020, a follow-up survey at each reintroduction site was conducted and additional DNA collected to assess whether genetic diversity was still maintained in the wild (based on survivorship).

The genetics
These samples were sent to MELFU for processing – across all our different cohorts, we had genetic samples for 256 fish. We used our genomics approaches – something regularly done for our fish projects – to generate over 8,000 genetic markers for assessment. This included 100 which we knew were linked to adaptation to hydroclimatic conditions, allowing us to differentiate between genome-wide and adaptive diversity in our data. Both types of diversity are crucial for population maintenance, particularly in terms of future adaptive capacity to changing environmental conditions. We used these genomic datasets to assess levels of genetic diversity and inbreeding; the amount of genetic mixing (admixture) in the populations; how related individuals were in each population, including identifying parents of the offspring and determining their reproductive contributions to the F1 population. Together, these analyses allowed us to assess different genetic aspects of the program to better understand its outcomes.
What did we find?
One of the first things we noticed was the strong genetic differentiation between our different source sites, including between the two Campaspe ones. Despite only being around 24 km away, these two populations were quite genetically distinct (FST = 0.53) and could be easily separated with the genetic data. This meant that the captive population of Campaspe individuals – which was a combination of these two sites – actually resulted in genetic mixing, with quite a few “hybrids” detected. This might sound like a problem – after all, part of the program design was trying to retain as much diversity from each of these populations as possible – but we also found an abundance of these “hybrids” in the recaptured population. In fact, around 28% of recaptured fish were “hybrids”, compared to around 17% of released fish: this might suggest that there is a benefit to mixing these populations (or at the very least, not a major barrier to it!).
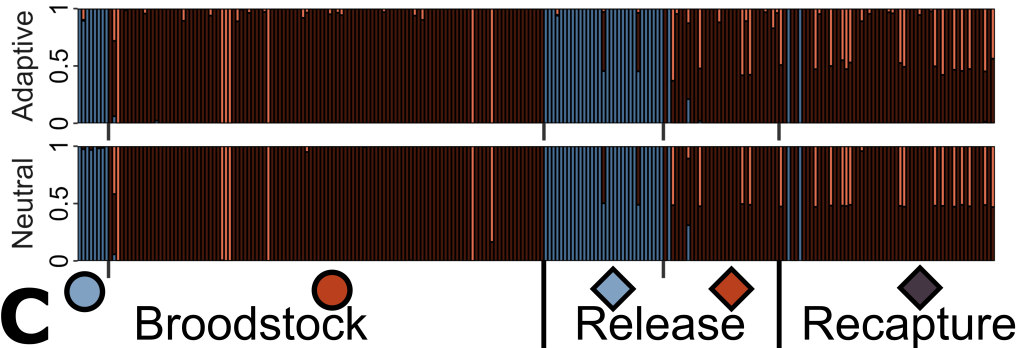
A key reason for that might be that our populations had very low genetic diversity to start – even by the generally depauperate standards of southern pygmy perch. When compared to populations from the lower reaches of the MDB (which was similarly the subject of a successful captive-breeding and reintroduction program), our source populations had around 30 – 60% less genetic diversity (depending on population and statistic). Under these circumstances, genetic mixing might be an essential strategy to boost genetic diversity, allowing these populations to persist and adapt over time: this is particularly important given that translocated populations will often lose genetic diversity over time through genetic erosion.
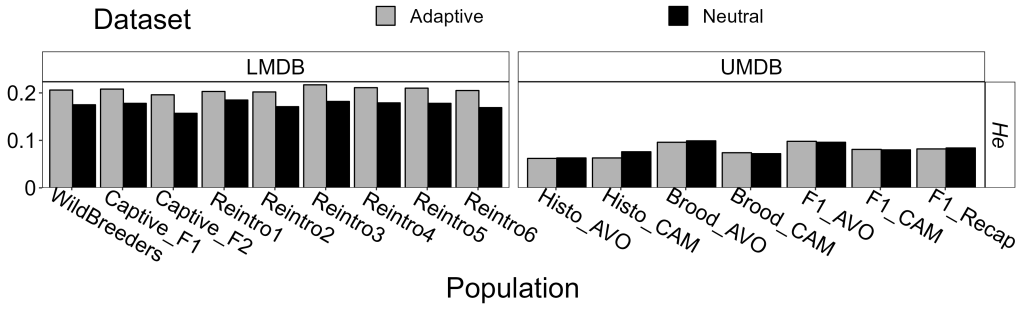
Despite these concerns, we did not find that the captive-breeding program itself worsened genetic diversity or inbreeding, with relatively unbiased reproductive output from our broodstock (i.e., the offspring weren’t overwhelmingly produced by a dominant male or female). Thus, we suggest that the efforts made by local communities did not jeopardise the genetic diversity of the reintroduced population, which is supported by subsequent monitoring showing large and healthy populations with an abundance of new, wild-bred fish across all release sites.
What did everyone get out of it?
Of course, this is great news for the fish: but what did the local communities gain from the collaboration? Well, the success of the reintroduction program has generated a framework for further similar programs: this includes collaborative reintroductions extended to new locations for southern pygmy perch, such as in Mildura (through collaboration with MCMA). Additionally, similar programs are now being designed and enacted for other freshwater fishes (e.g., southern purple-spotted gudgeon). To date, an additional seven landcare groups and over 20 individual landholders have been incorporated into new and ongoing reintroduction programs. Volunteer groups saw an increase in engagement and membership in response to the program as well, directly benefiting their networks. This included higher social media engagement; a series of outreach opportunities within the council area; and the formation of an educational program which provides southern pygmy perch and aquaria to local schools to teach students the importance of protecting native fishes, the impacts of pest species and basic water chemistry. Together, these highlight how collaborative efforts can not only lead to the best outcomes for conservation but provide tangible benefits to all members!

Southern pygmy perch are now being reintroduced into other sections of the Murray-Darling Basin, such as in Mildura. Source: Mallee Catchment Management Authority Facebook.
Community collaborations are essential for conservation
So, what can we learn from a program such as this? If anything, it’s to get involved! Local community groups and councils exist all across Australia, providing a way for direct involvement in your local area. Even more complex conservation actions, like reintroductions, can be facilitated through these networks with the right support and members: if you’re committed to seeing real and meaningful environmental change in your local area, check out what community groups exist and how your local council can support the environment. If you are planning something as ambitious as a reintroduction, I’d consider trying to contact a relevant (and hopefully local) university researcher: many of us are extremely passionate about practical conservation management and would love to try and help! Some of us might have access to resources or contacts that could really help ensure the program is as effective and informed as possible.
And, of course, no blog post about my favourite little fishes would be complete without a comment on taxonomic bias. Importantly, conservation across Australia is massively underfunded and under-resourced, and much of the support that exists tends to go towards large charismatic species (sorry mammals!). Many groups – including, but not limited to by any means, freshwater fishes – frankly don’t receive the support they need to persist. Without the monumental efforts and drive of the local communities here, conservation action for underappreciated species like southern pygmy perch would be missed. But your voice – and more importantly, your action – can provide a strong message and framework for the preservation of our forgotten biodiversity. Although many conservation messages are often bleak – stories of threats, declines and extinctions – our actions can help change the tide, even if it’s just in our own backyards. If, like me, you’re tired of the doom-and-gloom state of conservation in this country, channel that into direct, meaningful action: you might just change your own little corner of the world!
